 |
|
Information Box |
|
Trans-1,2-Dichloroethylene, Next generation solvent for
precision cleaning industry. To help the user
to escape from the continuous price increasing of n-Propyl
Bromide, Unistar is working on a new project to remove or
increase the flash point of 1,2-Trans. Candidates include
Methylene Chloride, HFC products. We have been successfully
increase the
flash point
to 50 degree C.
Our goal is to
create a affordable, steady supplying product to cleaning
industry.
|
|
 |
 |
|
Hot News |
|
n-PB price keep
jumping.
Because of the
shortage on bromine element, price of n-PB keep going up. It
breaks $2.00/lb recently. n-PB is losing its price
advantage.
Unistar will
expand its 1,2-trans production capacity to 5000MT/year. In
the new plant, a new production line of PERC will be setup
simultaneously. the expected production capacity of PERC
will be 10000MT/year
|
|
 |
|
|
Article Regarding Vapor Degreasing Solvent
Organic Solvent Cleaning
By
Wayne L. Mouser, Group Vice President of
Forward Technology
www.forwardtech.com
|
Vapor phase
solvent cleaning has been a mainstay
in the metal processing industries
since the early 1940's. Its
popularity was driven by the ability
to quickly remove organics such as
oils, greases, lubricants, coolants,
and resins in a single step. The
part, at ambient temperature, is
lowered into a solvent vapor. The
vapor, hotter than the part,
condenses on the part and dissolves
the organics. The part then is
withdrawn to the freeboard area
where the solvent evaporates from
the part, leaving it clean, spot
free, and dry. A vapor degreaser as
shown in Fig 1 was inexpensive to
own and operate.
During the early
1970's, use of the most popular
chlorinated solvent,
trichloroethylene(TCE), dropped from
a high of 609 million pounds in 1970
to a low of 90 million pounds in
1992. TCE fell out of favor due to
environmental issues such as ground
water pollution and air pollution
since TCE is a volatile organic
compound (VOC). Use of TCE was
partially replaced by
1,1,1-trichloroethane in the early
1970's and then by CFC-113 in the
late 1970's and 80's. |
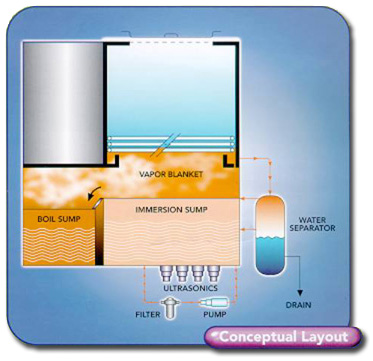
Fig. 1 |
|
Both CFC-113 and
1,1,1-tri were considered to be very
safe from a worker exposure point of
view. 1,1,1-tri was favored in the
metal working world while CFC-113
became the top choice in
electronics, aerospace, and many
other precision cleaning
applications. CFC-113 was effective,
low-cost, non-toxic, non-flammable,
and considered to be environmentally
preferable until the discovery of
the hole in the ozone.
In September
1987, the international community
signed an agreement to reduce the
usage of CFC-113 by 50%. At that
time, computer modeling suggested
that such a reduction would halt
theozone depletion. It was later
learned through more sophisticated
examination that a complete ban on
CFC-113 and 1,1,1-tri was needed to
protect the ozone layer. Worldwide
consumption of CFC-113 peaked at 279
million pounds in 1989 and was
banned in the U.S. in 1992.
1,1,1-triwas phased out at the end
of 1995.
The impending
loss of these two important solvents
started a frantic stampede by users
and suppliers to alternative
processes such as aqueous,
semi-aqueous, and alcohol based
processes. No clean fluxes and
soldering processes were developed
in the electronics industry. Metal
working primarily shifted to aqueous
cleaning and to no clean steps such
as vanishing oils. Precision
cleaning applications that could not
tolerate water switched to vapor
phase alcohol or engineered solvent
systems.
Today, a number
of new solvent choices have come on
the scene and recent advances in
equipment have allowed the safe use
of some of the older, more toxic,
solvents. The advantage to the user
is smaller foot print of the
equipment, faster throughput time,
and spot free finish produced by
vapor phase drying as opposed to
mechanical drying utilized in
aqueous processes.
The choice of the
right solvent to use is not an easy
one. Several factors must be
considered.
- Is the solvent compatible
with the substrate to be cleaned
and can it dissolve the
contaminant?
- Is the solvent and equipment
safe for the worker?
- Does the solvent and amount
used comply with local, state,
and federal regulations and
company policy?
- Does the solvent, process,
and equipment match the required
production rate and product
flow?
- Is the solvent and equipment
within budget?
In order to
choose a candidate solvent for a
specific process, it is helpful to
consider the physical properties.
Each of the solvents listed in Table
1 are commercially available and are
acceptable as vapor phase solvents.
This list is not inclusive by any
means. Several solvent suppliers
provide excellent solvents that are
an azeotrope or blend of the listed
solvents for specific purposes. As
an example, the HFC, HFE solvents
can be blended with trans-1,
2-dichloroethylene where additional
solvency is required. They are used
in a wide range of applications and
a review of the properties will
assist in choosing the right solvent
for a specific application. |
Table 1
|
Kauri-Butano
Value |
Boiling
Point °C |
Vapor
Density |
Surface
Tension Dynes/cm |
Vapor
Pressure Mm Hg 25 C |
Heat of
Vaporization cal/g |
|
Trichloroethylene |
129 |
87° |
4.53 |
28.7 |
70 |
56.4 |
|
Perchloroethylene |
90 |
121° |
5.76 |
32.3 |
20 |
50.1 |
| Methylene
Chloride |
136 |
39.8° |
2.93 |
27.2 |
350 |
78.7 |
| n-Propyl
Bromide |
125 |
71° |
4.25 |
25.3 |
111 |
58.8 |
| HCFC
(AK-225 AES) |
41 |
52° |
7 |
16.8 |
291 |
40.6 |
| HFC (Vertrel
XP) |
9.4 |
52° |
7.86 |
15.1 |
253 |
Tbd |
| HFE-71IPA |
10 |
54.8° |
7.51 |
14.5 |
207 |
39.5 |
| Acetone |
NA |
56° |
2 |
22.7 |
229 |
134.7 |
|
Cyclohexane |
58 |
80.7° |
2.9 |
24.9 |
95 |
85 |
| Isopropyl
Alcohol |
NA |
82° |
2.1 |
21.7 |
40 |
166.1 |
| N-Methyl
Pyrrolidone (NMP) |
350 |
204.3° |
3.4 |
40.7 |
0.24 |
127.3 |
| D-Limonene |
67 |
154° |
4.73 |
25 |
2 |
NA |
| Trans-1,
2-Dichoroethylene |
117 |
47.8° |
3.34 |
27.5 |
330 |
72 |
|
|
The Kauri-Butanol
Value is a measure of solvency
power. It is important to choose a
KB value that matches the
contaminant to be removed and at the
same time does not affect the
substrate. An extreme example would
be removal of epoxy.About the only
solvent strong enough to attack
epoxy is N-Methyl Pyrrolidone (NMP).If
the substrate were steel, NMP would
be acceptable but if the substrate
were aprinted wiring assembly, NMP
would be too aggressive. Generally
the higher KB value solvents are
used for heavy organics such as oils
and greases. A good example
iscyclohexane that excels in removal
of rosin flux, oils, and heavy
greases. Lower KBvalue solvents are
used in critical cleaning where
particle removal and light organics
are found.
KB values are not
available for acetone and IPA.
Practical experience indicates that
acetone is a mild selective solvent
best used for adhesive or mild
organic removal. Cyclohexane excels
in removal of rosin flux, oils, and
heavy greases. IPA is the mildestof
the three and is used extensively in
critical cleaning for electronics,
medical implants, aerospace, disk
drive, or where a spot free
hydrophilic surface is required.
The Boiling
Point is important when the
contaminant is temperature sensitive
such as wax or when the substrate is
sensitive to temperature. An example
would be removal of wax with
Perchloroethylene with a boiling
point of 121 degrees C. Most waxes
will melt and degrease nicely at
that temperature but some substrates
could not tolerate that
temperature.It is also important to
consider the purity of the vapor
blanket in the machine. The higher
the boiling point, the more
likelihood of organic concentration
in the vapor. This could lead to
organic contamination of the
substrate during the drying phase.
Vapor Density
is a measure of the weight of the
vapor blanket where air = 1. Allof
the selected solvents are heavier
than air. That is good. It helps
keep the solvent in the machine.
Emissive solvent loss is a function
of the boiling point and the vapor
density. Also proper equipment
design can minimize emissive loss.
Surface
Tension is the storage of energy
at the surface of liquids. Surface
tension tries to minimize surface.
Imagine a droplet of water on a flat
surface. The high surface tension of
water (78) causes the water to form
a bead. This force makes it
difficult for water to penetrate
tight crevices. The surface tension
of the selected solvents is
muchlower. A similar sized droplet
of any of the solvents would spread
over the surface rather than bead
up. The solvent is able to creep
into tight spaces, dissolve
contaminate, and then be flushed out
with fresh solvent. The lower the
surface tension, the better.
Vapor Pressure
measures the pressure the vapor
exerts over the liquid at
equilibrium. This physical
characteristic determines what
solvents can be used as the sole
solvent in vapor phase cleaners.
Note that NMP and D-limonene have
low vapor pressures. They also have
high boiling points and cannot be
easily converted from liquid to
vapor. Both are excellent solvents,
but should only be considered in
co-solvent systems where a second
solvent such as isopropyl alcohol
can be used as the rinsing and vapor
phase portion of the cleaning cycle.
Heat of
Vaporization is the measure of
energy necessary to convert liquid
to vapor at the boiling point. Note
that most of the chosen solvents
have a much lower heat of
vaporization than the energy
required for water (539 cal/gm).
These solvents are much easier to
dry from the part as compared to
water. That means quicker drying
times and lower energy costs.
Once candidate
solvents have been chosen for
solvency and compatibility, the next
step is to evaluate the solvent and
equipment for worker safety and
regulatory compliance. There are
many methods of evaluation and each
user must determine the degree of
importance of various safety issues
and also must comply with local
requirements as well as company
dictated requirements. Some
important safety concerns are listed
in Table 2. |
Table 2
|
HAPs |
TLV |
Flash
Point Closed Cup °C |
Total
Hazard Value |
|
Trichloroethylene |
Yes |
50 |
None |
41.3 |
|
Perchloroethylene |
Yes |
25 |
None |
37.5 |
| Methylene
Chloride |
Yes |
25 |
None |
30.1 |
| n-Propyl
Bromide |
No |
100* |
None |
9.2 |
| HCFC
(AK-225 AES) |
No |
50 |
None |
5.2 |
| HFC (Vertrel
XP) |
No |
213** |
None |
12.7 |
| HFE-71IPA |
No |
750/400 |
None |
7.3 |
| Acetone |
No |
750 |
-17 |
15.9 |
|
Cyclohexane |
No |
300 |
-20 |
23.5 |
| Isopropyl
Alcohol |
No |
400 |
0.6 |
14.2 |
| N-Methyl
Pyrrolidone (NMP) |
No |
100 |
93 |
7.1 |
| D-Limonene |
No |
NE |
49 |
7.8 |
| Trans-1,
2-Dichoroethylene |
No |
200 |
2.2 |
28.6 |
|
|
|
* Supplier recommendation |
|
** Supplier calculated AEL |
|
|
HAPs Title
III of the 1990 Clean Air Act
Amendments lists 189 compounds that
are classified as hazardous air
pollutants (HAPs). At the very
least, the HAPs solvents must be
used in a NESHAP compliant machine
and their emission to the atmosphere
must be tightly controlled. Due to
their known toxicity and potential
damage to the environment, it is the
opinion of this writer that HAPs
solvents should only be used in
airtight machines.Many companies
have made the decision to move to
alternate solvents to avoid the
complications of dealing with HAPs
solvents.
TLV.
Threshold Limit Values are
guidelines established by the
American Conference of Governmental
Industrial Hygienists (ACGIH) to
assist industrial hygienists in
making decisions regarding safe
levels of exposure to various
hazards found in the workplace. TLV
reflects the level of exposure that
the worker can experience without an
unreasonable risk of disease or
injury. A low TLV number should
suggest the solvent be used in an
airtight machine or that the
workplace and worker be carefully
monitored so that the exposure level
does not exceed the TLV.
Flash Point.
Any solvent listed with a flash
point should only be used in
equipment properly designed for low
flashpoint or combustible solvents.
There are many advantages to taking
this route. Solvents such as IPA,
cyclohexane, and acetone are
excellent solvents relative to their
physical properties and low
toxicity. They clean well and
produce a spot free finish. They are
easy to dispose of and are
inexpensive, actually cheap when
compared with other alternatives.
The disadvantage is since they are
flammable; they require more
expensive equipment to safely
operate.
Total Hazard
Value. There are many methods of
evaluating the safety of solvents.
TLV, VOC, HAPs, PEL, AEL, ODP to
name a few. The list goes on and on.
If you have an environmental, health
and safety staff, they are no doubt
experts in this field. If you need
help, you might want to consider a
rating system called the Indiana
Relative Chemical Hazard Score (IIRCHS)developed
by the
Clean Manufacturing Technology and
Safe Materials Institute (CMTI)
located at Purdue University. If you
don't find the chemistry you are
interested in, give Shayla Barrett,
Process Engineer, a call. Shayla was
very helpful in the preparation of
this paper.
The formula for
the assignment of the total hazard
value covers many health and
environmental concerns. An excellent
reference is an article entitled
"Solvent by the Numbers" by Charlie
Simpson published in the January
2002 CleanTech magazine. |
|
Now that solvent
compatibility and safety have been
addressed, the next step is to
consider process flow and production
capacity. This will determine the
type of equipment you will need.
Product offerings start with manual
open top designs (Fig 2).

|
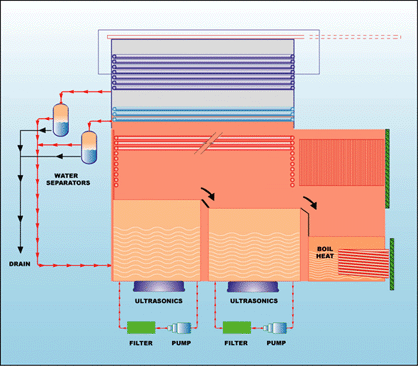
Fig. 2 |
|
To fully
automated machines capable of
cleaning a basket of parts every
couple minutes (Fig.3). |

Fig. 3 |
|
The solvent of
choice will also impact the
equipment decision. Some of the
solvents are acceptable to use in
open top manual machines, while
other solvents, because of their
environmental and worker safety
concerns, are best used in air tight
or vacuum machines (Fig. 4). |
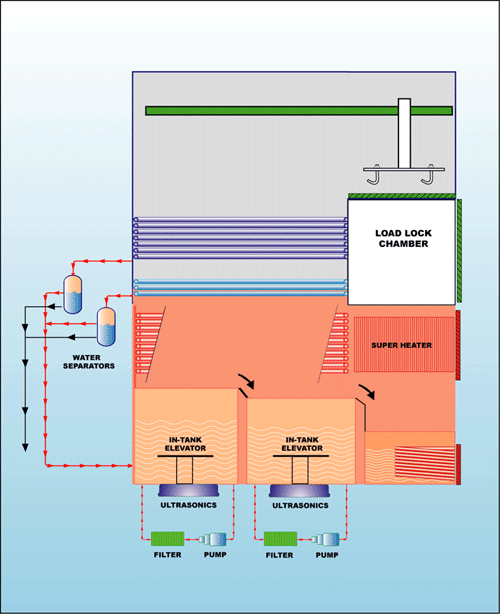
Fig. 4 |
|
Low flash point
solvents require equipment (Fig. 5)
built specifically to address
flammability issues. They are
compatible with acetone, cyclohexane,
IPA, NMP, D-Limonene, as well as the
other listed solvents that do not
exhibit a flash point. |

Fig. 5 |
| The final step is
the all-important budget for the
project. A solvent cost comparison
appears in Fig.6. |
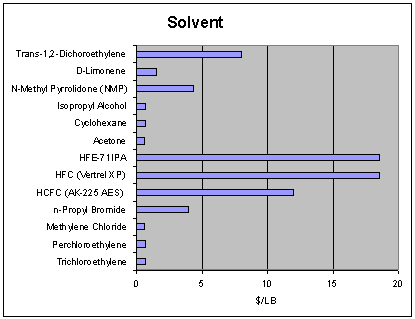
Fig. 6 |
|
Equipment cost
varies with the size, materials of
construction, cleaning cycle, and
degree of automation. Prices can
range from less than $10,000 to over
$1,000,000.
Vapor phase
solvent cleaning is an important and
valuable tool. This cleaning process
offers complete washing, rinsing,
and drying in a small footprint
while minimizing energy, floor
space, and process time. With
today's efficient equipment and safe
solvents, the process is expected to
flourish. |
|
| |
| |
|
|

